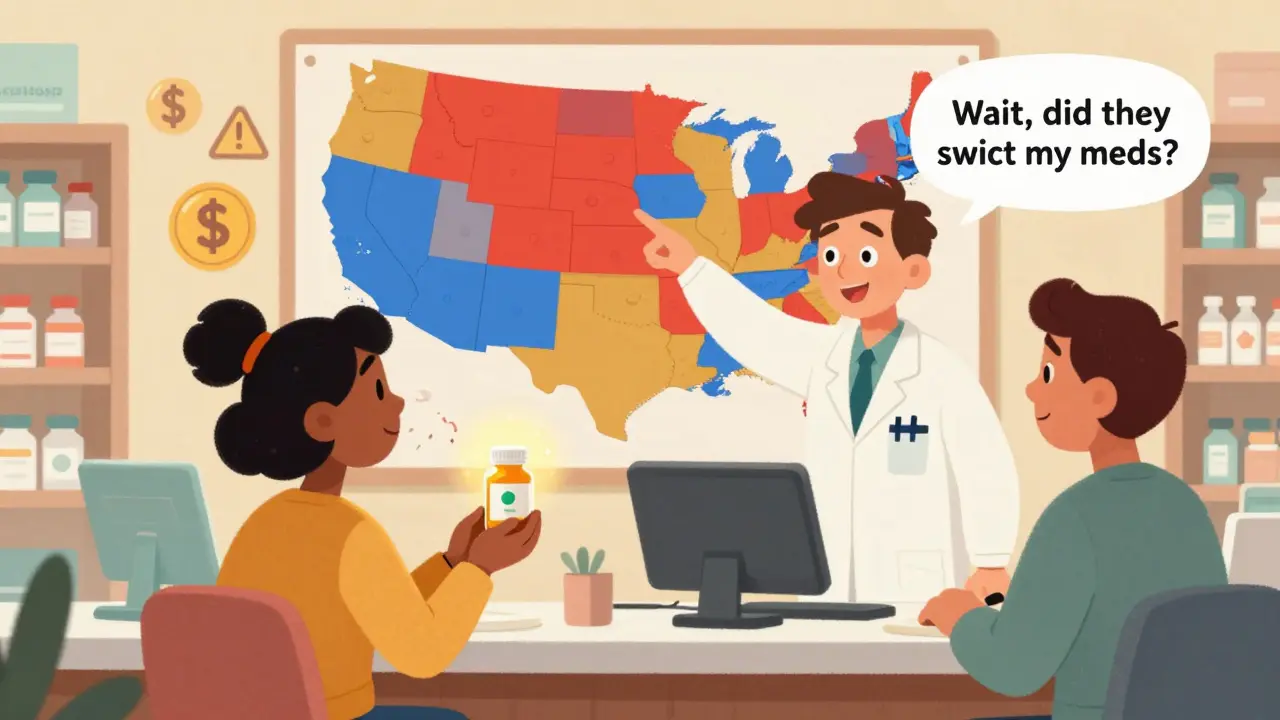
When you pick up a prescription, you might not think about whether the pill in the bottle is the brand name or a generic version. But behind that simple swap is a complex web of state laws that can mean the difference between saving money and facing confusion-or even risk. In some states, pharmacists are required to swap a brand-name drug for a cheaper generic unless the doctor says no. In others, they can’t substitute at all without your explicit permission. And when it comes to biologics-complex drugs used for conditions like rheumatoid arthritis or cancer-the rules get even more tangled. With 50 states and Washington, D.C., each setting their own rules, pharmacists are forced to juggle a patchwork of conflicting regulations every single day.
How Generic Substitution Laws Work
Generic drugs are chemically identical to their brand-name counterparts. They work the same way, have the same side effects, and are approved by the FDA. The only real difference? Price. Generics cost, on average, 80% less. That’s why federal law encourages substitution-but it leaves the actual rules to the states. The foundation for this system was laid in 1984 with the Hatch-Waxman Act, which created a fast-track approval process for generics. But after that, each state went its own way. Today, 19 states have mandatory substitution laws. That means if a prescription is written for a brand-name drug and a generic is available and rated equivalent by the FDA’s Orange Book, the pharmacist must dispense the generic-unless the prescriber writes “dispense as written” or the patient refuses. The other 31 states and D.C. operate under permissive substitution. Here, pharmacists can substitute, but they’re not required to. Many of these states require the pharmacist to notify the patient before swapping, or even get their consent. In Oklahoma, you need written authorization from either the prescriber or the payer before any substitution can happen. That’s rare. Most states don’t go that far.The Big Split: Small-Molecule vs. Biosimilars
Not all generics are the same. Traditional generics are small-molecule drugs-think blood pressure pills, antibiotics, or antidepressants. Biosimilars are different. They’re copies of biologic drugs, which are made from living cells. Think insulin, rheumatoid arthritis treatments, or cancer therapies. These aren’t exact copies. They’re highly similar, but tiny differences can matter. Because of that, states treat biosimilars differently. As of 2023, 45 states (plus D.C.) have stricter rules for biosimilar substitution than for small-molecule generics. In most of these states, pharmacists must notify the prescribing doctor within a set time frame-often 5 days-after substituting. California requires that notification to be made electronically and be accessible to the prescriber. Other states require patient consent before substitution, or even mandate that the biosimilar be labeled differently on the bottle. Only six states treat biosimilars the same way as regular generics. That’s a problem. Patients switching from a brand biologic to a biosimilar may not realize the change happened. And if something goes wrong, it’s harder to trace whether the issue came from the drug or the substitution itself.Who Gets to Say No? Patient Consent and Prescriber Rights
Patient autonomy sounds great in theory. But in practice, only 7 states and D.C. require pharmacists to get your explicit consent before swapping a drug. In the other 43 states, you might never know your medication changed unless you check the label. That’s a problem for people on narrow therapeutic index drugs-like warfarin, lithium, or thyroid meds-where even tiny differences in absorption can cause serious side effects. Prescribers have more control. In all 50 states and D.C., a doctor can write “dispense as written” or “do not substitute” on the prescription. That’s a legal barrier. Some doctors do it out of habit. Others do it because they’re worried about variability. A 2021 study by the Institute for Safe Medication Practices found that 22% of substitution-related medication errors happened in states with weak notification rules. That’s why some prescribers just say no to avoid risk. But here’s the catch: pharmacists in 24 states have no legal protection if something goes wrong after a substitution. If a patient has an adverse reaction, the pharmacist could be sued-even if they followed every state rule. That’s why some pharmacists refuse to substitute, even when it’s allowed. Sarah Jennings, a pharmacist in Connecticut, told Pharmacy Times she won’t swap warfarin because she doesn’t want to be liable if the patient’s INR levels go haywire.
What Pharmacists Actually Deal With
If you’ve ever worked in a pharmacy, you know the paperwork doesn’t stop at filling prescriptions. In states with complex substitution laws, pharmacists spend an average of 8.2 hours a month just reviewing rules. That’s not time spent counseling patients. It’s time spent checking state databases, confirming formulary lists, and making sure electronic health records are synced. Only 28 states have fully integrated substitution rules into major EHR systems like Epic or Cerner. That means in 23 states, pharmacists still have to manually look up each state’s requirements. One pharmacist on Reddit said they spend 15 to 20 minutes a day just checking rules for multi-state telepharmacy work. The National Community Pharmacists Association found that 41% of pharmacists made at least one substitution error in the past year because of confusing rules. The burden hits independent pharmacies hardest. Chain pharmacies have legal teams and software systems to track changes. Independent pharmacies? They rely on free resources like the National Association of Boards of Pharmacy’s website. But 53% of pharmacists say even that lacks enough detail for biosimilars. A 2023 survey showed independent pharmacies were 60% more likely to report substitution errors than chain pharmacies.Where the System Works Best
Some states have figured out how to make substitution smooth and safe. California stands out. Since 2022, they’ve required electronic notification to prescribers for biosimilar substitutions. That system cut substitution errors by 32%. Florida and New York have clear, standardized formularies that list which generics can be swapped without confusion. In those states, pharmacists spend less time on paperwork and more time on patient care. The key? Consistency. When the rules are simple, clear, and backed by technology, adoption goes up. States with mandatory substitution laws have generic utilization rates of 85.3%, compared to 76.6% in permissive states. That’s not just about savings-it’s about access. Patients are more likely to take their meds when they’re affordable.The Push for Change
The current system is unsustainable. Pharmacy chains spend an average of $1.2 million per state annually just to stay compliant. Nationally, that’s $61.2 million a year-money that could be spent on patient care. The National Association of Boards of Pharmacy launched a project in January 2024 to reduce 51 different state laws to just three regional models by 2026-2027. The FDA is also pushing for alignment, calling state fragmentation the “single greatest barrier” to biosimilar adoption. In 2023 and 2024, 17 states introduced reform bills. Nine of them passed laws to bring biosimilar rules closer to small-molecule rules. Texas, Illinois, and Pennsylvania are leading the way. But change is slow. The Congressional Budget Office estimates harmonized laws could save $14.3 billion over 10 years. Yet 72% of pharmacy leaders say they support federal preemption-overriding state laws with a national standard. The question isn’t whether substitution should happen. It’s whether we’re willing to make it simple enough for pharmacists to do it right.
What Patients Should Know
You have rights. If you’re on a medication that’s critical-like blood thinners, seizure drugs, or insulin-ask your pharmacist: “Is this the same as what I was taking before?” Check the label. If it’s a biosimilar, it should say so. If you’re not told about a switch, you have every right to ask why. In states with consent requirements, you can say no. In states without them, you can still ask for the brand name-even if it costs more. Talk to your doctor. If you’ve had side effects after a switch, report it. Your feedback helps improve the system.What’s Next for Generic Substitution
The future of substitution depends on three things: technology, standardization, and trust. Real-time updates in pharmacy software are already reducing errors. But without consistent rules across states, even the best tech can’t fix the confusion. Biosimilars are the next frontier. With over 30 approved and many more in development, we need a system that treats them with the same clarity as generics. Right now, we’re treating them like they’re something entirely new-when they’re really just the next step in lowering drug costs. The goal isn’t to eliminate brand-name drugs. It’s to make sure patients get the right medicine at the right price-with no surprises.Can a pharmacist substitute my brand-name drug without telling me?
In 20 states and D.C., pharmacists are not required to notify you before substituting a generic drug. In the other 31 states and D.C., they must inform you-either verbally, in writing, or electronically. Always check the label and ask if you’re unsure. If you’re on a drug with a narrow therapeutic index, like warfarin or lithium, you should always confirm the substitution with your pharmacist or prescriber.
Do I have to give permission to switch to a generic drug?
Only in 7 states and D.C. is explicit patient consent legally required. In most places, the pharmacist can substitute without asking-if the generic is FDA-approved and the prescriber didn’t block it. But you can always request the brand name, even if it costs more. Your right to choose doesn’t disappear just because substitution is allowed.
Are biosimilars treated the same as regular generics?
No. In 45 states and D.C., biosimilars have stricter rules than small-molecule generics. Pharmacists often must notify your doctor after substitution, and some states require your consent. Biosimilars are not exact copies of biologics-they’re highly similar, but subtle differences matter. That’s why states treat them differently, even though the FDA considers them safe.
What happens if I have a bad reaction after a generic substitution?
Liability depends on your state. In 24 states, pharmacists have no legal protection if something goes wrong after a substitution-even if they followed all rules. In 26 states, they’re shielded from liability as long as they complied with state law. If you experience side effects after a switch, report it to your pharmacist and doctor immediately. You can also file a report with the FDA’s MedWatch program.
How do I know if my drug was substituted?
Check the label. The generic name will be listed first, followed by the brand name in parentheses. For biosimilars, the label must include the word “biosimilar” or “interchangeable.” If you’re unsure, ask the pharmacist. Many pharmacies also include a sticker or note on the bottle. Don’t assume-always verify.
Why do some doctors refuse to allow substitutions?
Some doctors write “dispense as written” out of habit, fear of variability, or past experience with side effects. Others are unaware that generics are required to meet strict FDA standards. In states with poor notification systems, doctors may not even know a substitution occurred. This is changing as more states adopt electronic alerts, but many prescribers still default to blocking substitution to avoid risk.
Can I ask for the brand-name drug even if a generic is available?
Yes. You have the right to request the brand-name version, even if a generic is available and allowed under state law. Your pharmacist may need to contact your insurance to see if the brand is covered, or you may have to pay the full price out of pocket. But you can’t be forced to take a generic if you don’t want it.
Are there tools to help pharmacists keep up with state rules?
Yes. The National Association of Boards of Pharmacy offers a free online database updated quarterly. Some pharmacy software systems, like ScriptPro SP 200, now include real-time state-specific substitution rules. These tools reduce errors by up to 37%. However, many independent pharmacies still rely on manual checks, which increases the risk of mistakes.



9 Comments
This whole system is a fucking mess. Pharmacists are expected to be lawyers, scientists, and customer service reps all at once, and no one gives a shit about the burnout. I’ve seen people get prescribed insulin, get a biosimilar swapped in without a word, and then end up in the ER because their blood sugar went nuts. And the worst part? The pharmacist gets sued, not the state that let this shit happen.
There’s a profound irony in how we treat pharmaceutical substitution: we demand cost-efficiency while simultaneously refusing to harmonize the regulatory landscape that enables it. The fragmentation of 51 distinct legal regimes isn’t just inefficient-it’s epistemologically incoherent. We’ve outsourced public health policy to state legislatures with no pharmacological training, no clinical insight, and no accountability. The result is a patchwork of arbitrary constraints that prioritize bureaucratic inertia over patient safety. If we truly valued equity in healthcare, we’d recognize that a diabetic in Alabama deserves the same clarity as one in Oregon. The solution isn’t more paperwork-it’s a national standard grounded in evidence, not political expediency.
I work in a pharmacy and this is 100% accurate. We spend more time checking state laws than talking to patients. I had a lady come in yesterday asking why her thyroid med looked different. I had to pull up three different state databases, call her doctor’s office, and then explain the difference between generic and biosimilar. She was confused, I was exhausted. We need better tech and fewer rules. Not more.
Stop making pharmacists guess. Just make one rule.
so i just found out my insurance switched my biologic without telling me?? i had no idea. i’ve been feeling off for weeks and thought it was stress. now i’m scared. anyone else have this happen? i’m in texas and they didn’t even send a letter. this feels so wrong.
ohhhh so that’s why my pharmacist in india (yes, i’m in india but using a us telepharmacy) kept asking me 17 questions before filling my rheumatoid arthritis med. i thought he was being extra nice. turns out he was doing state-by-state legal research from a phone in bangalore. bless his soul. but seriously, if we can send rockets to mars, why can’t we have one database that says ‘this drug can be swapped here’? it’s 2024, not 1994.
wait so if i’m on warfarin and they swap me to a generic without telling me… is that even legal? i thought generics were fine, but warfarin’s different right? i’m gonna check my next bottle. 🤔
of course california’s the only one doing it right. because they’re always the ones trying to ‘fix’ everything. meanwhile, real states like Texas and Florida are just letting pharmacists do their damn jobs without 17 forms and a notary. this is why america’s falling apart-overregulated liberals trying to control every pill bottle. just let people choose! if i want the brand name, i’ll pay for it. stop making me jump through hoops because some bureaucrat in Sacramento is scared of biosimilars.
my uncle in Ontario just got switched to a biosimilar and didn’t know until his bloodwork came back weird. he called the pharmacy screaming. they said ‘oh, we’re allowed to do that here.’ he’s not in the U.S., but this is the same damn mess. we need a global standard. how is it possible that one country has 51 rulebooks for the same damn pill? this isn’t healthcare-it’s a bureaucratic circus. someone get the clowns out of the pharmacy.