When a blockbuster drug’s patent runs out, generics should flood the market and slash prices-often by 80% or more. But in many cases, they don’t. Why? Because pharmaceutical companies have spent years building a legal shield around their drugs, not with breakthrough science, but with tiny tweaks and patent filings that have nothing to do with better patient outcomes. This is evergreening: the deliberate strategy of extending patent life by making minor changes to existing drugs, blocking generics, and keeping prices high.
How Evergreening Works in Practice
Every new drug gets a 20-year patent clock. But that’s not the full story. Once the drug is approved by the FDA, companies can stack on extra layers of protection-sometimes adding decades of exclusivity. These aren’t new medicines. They’re the same active ingredient, just packaged differently: a new pill shape, a slow-release version, a combo with another drug, or a switch from prescription to over-the-counter. The active ingredient? Still the same. The benefit to patients? Often negligible. The profit boost for the company? Massive.
Take AstraZeneca’s Prilosec, a heartburn drug. When its patent neared expiration, the company launched Nexium-a slightly modified version with a different chemical form. Nexium wasn’t more effective. In fact, studies showed it worked no better than the original. But it came with a new patent. And a new price tag. Within a year, Nexium was outselling Prilosec. The reason? Doctors kept prescribing it, insurers kept covering it, and patients didn’t know the difference. That’s not innovation. That’s marketing dressed up as medicine.
The Patent Thicket Strategy
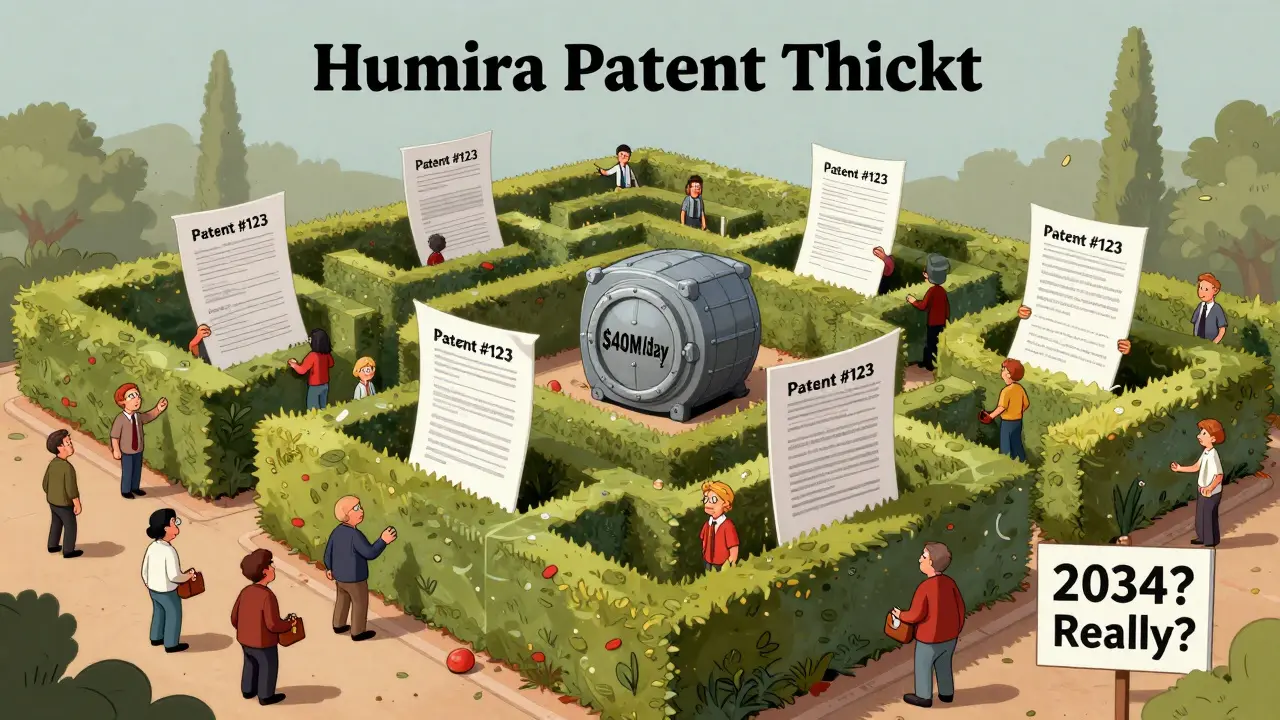
One patent isn’t enough. Companies like AbbVie, maker of Humira, file dozens-sometimes hundreds-of patents around a single drug. Humira, used to treat autoimmune diseases like rheumatoid arthritis, has over 247 patents tied to it. More than 100 have been granted. This isn’t an accident. It’s a calculated tactic called a “patent thicket.”
Imagine a hedge maze made of legal documents. Every time a generic manufacturer tries to enter the market, they hit a new patent. Each one requires a lawsuit. Each lawsuit costs millions. Most generics can’t afford to fight all 247. So they wait. And wait. And wait. By the time one patent expires, another one kicks in. AbbVie extended Humira’s market exclusivity past 2034-nearly 20 years beyond the original patent. In 2023, Humira was still generating $40 million in daily revenue. That’s not luck. That’s strategy.
Types of Evergreening Tactics
There’s no single way to evergreen a drug. Companies use a toolkit of tricks, many of them legal but ethically questionable:
- New dosage forms: Switching from a tablet to a liquid, or from daily to weekly dosing. The drug’s effect doesn’t change, but the patent does.
- Combination products: Bundling an old drug with a new one-even if the new one is cheap and widely available. The combo gets a fresh patent.
- Orphan drug status: Claiming a drug treats a rare disease, even if it’s widely used for common conditions. This grants seven years of exclusivity.
- Pediatric exclusivity: Running small studies on children, even if the drug has no known benefit for them. This adds six months.
- Product hopping: Pulling the original drug off the market and pushing patients toward the “new” version. No more generics for the old one. Only the new, patented version is available.
- Authorized generics: The brand company itself launches a generic version-just cheaper. This crushes independent generics before they even start.
These aren’t theoretical. They’re documented in court filings, FDA records, and internal company documents. Harvard researchers found that 78% of new patents for prescription drugs are for existing drugs-not new ones. That means most patent activity isn’t about innovation. It’s about extension.
Why This Hurts Patients and Systems
Generic drugs aren’t just cheaper. They’re just as safe and effective. The FDA requires them to meet the same standards as brand-name drugs. But evergreening blocks them. And that has real consequences.
Patients with chronic conditions like diabetes, Crohn’s disease, or psoriasis are stuck paying hundreds or even thousands of dollars a month. In the U.S., where drug prices are among the highest in the world, this leads to rationing-people skipping doses, splitting pills, or going without. A 2023 WHO report called evergreening a “major barrier” to medicine access in low- and middle-income countries. In Australia, where public drug subsidies exist, patients still face higher co-pays when generics are delayed.
Health systems pay the price too. Medicare, Medicaid, and private insurers spend billions more each year because of evergreening. The Inflation Reduction Act of 2022 tried to fix this by letting Medicare negotiate prices for the top 10 most expensive drugs. But if the drug never goes generic, negotiation doesn’t help much. The system is still rigged.
Who Benefits? Who Loses?
Pharmaceutical companies win. AstraZeneca extended patent life by over 90 years across just six drugs. AbbVie made $160 billion from Humira alone before generics could enter. These are not small gains. They’re corporate windfalls built on legal loopholes.
Patients lose. Taxpayers lose. Health systems lose. Even doctors lose-they’re forced to prescribe more expensive options even when cheaper, equally effective alternatives exist.
Meanwhile, the cost to develop a truly new drug is around $2.6 billion and takes 10-15 years. Evergreening? A fraction of that cost. A chemist tweaks a molecule. A lawyer files a patent. A marketer rebrands the pill. Profit. No clinical breakthrough. No new science. Just a longer monopoly.
Is There Any Pushback?
Yes. And it’s growing.
In 2022, the U.S. Federal Trade Commission sued AbbVie, calling Humira’s patent strategy “anticompetitive.” The FTC argued that the 247 patents were a deliberate obstruction-not innovation. Courts are starting to pay attention. In 2023, a judge in California blocked a product hop by a major drugmaker, ruling the switch was “not for therapeutic benefit but to delay generics.”
The European Medicines Agency now requires proof of “significant clinical benefit” before granting extra exclusivity. India and Brazil have blocked evergreened patents outright. Canada has tightened its rules on combination drugs.
But the industry fights back. Lobbying spends billions each year to protect the status quo. And with biologics-complex, hard-to-copy drugs-on the rise, the next wave of evergreening is already here. Companies are patenting delivery systems, packaging, and even genetic tests that predict who responds to the drug. These aren’t improvements. They’re barriers.
What Can Be Done?
There’s no single fix, but progress is possible:
- Stronger patent review: The USPTO needs to reject obvious or trivial modifications. Patents should require real innovation, not minor tweaks.
- Limiting exclusivity stacking: No more adding six months for pediatric studies if the drug has no pediatric use. No more seven years for orphan status if the drug treats millions.
- Transparency: All patent filings tied to a drug should be publicly listed and tracked. No hidden thicket.
- Fast-tracking generics: The FDA needs more resources to approve generics faster and challenge patent abuse.
- Price caps: If a drug has no new benefit, its price should be capped at generic levels-even before generics arrive.
Some countries are already doing this. The result? Lower prices. Faster access. More patients treated.
What Patients Should Know
If you’re on a brand-name drug, ask your doctor: Is there a generic? If not, why? Sometimes, the answer is simple: the generic exists, but your insurer won’t cover it because the brand company paid them to block it. Sometimes, the brand just switched to a new version-and the old one vanished from shelves. That’s product hopping.
Don’t assume your prescription is the best option. Ask about cost. Ask about alternatives. Ask if the drug you’re taking has been on the market for more than 10 years. If yes, and there’s no generic, there’s a good chance evergreening is at play.
And if you’re a caregiver, advocate, or patient group leader-push for change. Demand transparency. Support policy reform. The system isn’t broken. It was built this way.
Is evergreening illegal?
Not usually. Evergreening exploits legal loopholes, not outright laws. While some tactics have been challenged in court and ruled anticompetitive, most are still allowed under current patent and FDA rules. The system was designed to encourage innovation, but it’s being used to delay competition.
Do evergreened drugs work better than generics?
Almost never. Studies consistently show that modified versions-like Nexium over Prilosec or new formulations of Humira-offer no meaningful clinical benefit. The changes are chemical or delivery-based, not therapeutic. Patients get the same effect, just at a higher price.
How long can a drug’s exclusivity last because of evergreening?
While the original patent lasts 20 years, evergreening can extend total exclusivity to 30, 40, or even more than 50 years. Humira’s protections are expected to last until 2034-nearly 25 years after its initial approval. That’s not the norm, but it’s becoming more common.
Can I get a generic version of my drug if it’s been evergreened?
Maybe-but not always. If the original version is still on the market, a generic may be available. But if the company pulled it and only sells the new version, you’re stuck. Some insurers will cover the generic if you ask, but others won’t. Always check with your pharmacist and ask for the original active ingredient.
What’s the difference between evergreening and real innovation?
Real innovation introduces a new molecular structure, a new target, or a proven breakthrough in treatment. Evergreening changes the pill-its shape, timing, or combo-but not the medicine inside. One saves lives. The other saves profits.
What’s Next?
The battle over evergreening is far from over. As biologics and gene therapies become more common, the stakes are rising. These drugs are harder to copy, making them prime targets for patent extensions. But public pressure is growing. Patients are speaking up. Legislators are listening. And courts are starting to question the logic of paying $10,000 a year for a drug that’s been around for 15 years with no real improvement.
Change won’t come overnight. But every time a patient asks, “Why can’t I get this cheaper?”, every time a doctor prescribes a generic, and every time a regulator blocks a trivial patent, the system moves a little closer to fairness.







11 Comments
Every time I fill a prescription, I wonder if I’m paying for science or legal loopholes. This post laid it out so clearly-no fluff, just facts. It’s not just about money; it’s about dignity. People skipping doses because they can’t afford the brand? That’s not healthcare. That’s systemic cruelty.
And yet, we keep applauding pharmaceutical CEOs as innovators. When did we decide that rebranding a pill counts as progress?
They’re not just evergreening-they’re running a global scam. The FDA, the USPTO, Congress-all bought and paid for. You think Humira’s patents are accidental? No. It’s a coordinated war on public health. The same people who fund political campaigns also write patent laws. And you wonder why insulin costs $300 in America and $3 in India?
Wake up. This isn’t capitalism. It’s corporatist feudalism.
Thank you for this meticulously researched piece. 🙏
As someone from India, where generic drugs are the lifeline for millions, I find it deeply troubling that such practices are normalized in wealthier nations. The ethical implications extend far beyond profit margins-they touch human survival.
Perhaps we need a global coalition to redefine what constitutes 'innovation' in pharmaceuticals. Not just legally, but morally.
Let’s be precise: evergreening is a textbook violation of the non-obviousness requirement under 35 U.S.C. §103. The USPTO is failing its statutory mandate by granting patents for trivial modifications-like changing the salt form or switching from tablet to capsule. These aren’t inventions; they’re administrative arbitrage.
And the FTC’s lawsuit against AbbVie? Long overdue. The legal theory here is rock-solid: exclusionary conduct under §2 of the Sherman Act. This isn’t about morality-it’s about antitrust enforcement.
OMG. I just read this and I’m shaking. 😭
My mom’s been on a brand-name arthritis drug for 12 years-same exact molecule as the $5 generic, but they pulled the old version off shelves and now she pays $1,200/month. She cries every time she swallows that pill. And the pharmacy? Says they ‘can’t substitute’ because ‘it’s not the same.’
It’s the same damn drug. They just made it shinier and more expensive. This isn’t medicine. It’s a heist.
Who do we sue? Who do we protest? I’m so mad I could scream.
Wait-so you’re telling me the entire pharmaceutical industry is built on lies? That every time I see an ad for a ‘miracle’ new drug, it’s just the same old stuff in a different color?
I feel so betrayed. I trusted these companies. I believed their ads. I thought they cared. And now I find out they’re just… manipulating us?
My whole life, I thought science was noble. Now I don’t know what to believe anymore.
My uncle in Delhi takes a generic version of a drug that costs $200 a month in the US. Same active ingredient. Same results. He’s alive because of it. Here, people die because of profit margins. It’s not hard to fix. Just stop letting lawyers run medicine.
This is one of the most important public health issues no one talks about. I’ve worked in community clinics for 15 years. I’ve seen patients choose between insulin and groceries. I’ve watched elderly people split pills in half because they can’t afford the full dose.
Evergreening isn’t just a corporate tactic-it’s a public health crisis disguised as intellectual property law.
We need to treat this like we treat tobacco or opioids: with urgency, transparency, and moral clarity.
In India, we call this ‘patent trolling.’ Big pharma comes here, copies our generics, then sues us for making cheap medicine. Meanwhile, they sell the same drug for 10x in the US.
It’s colonialism with a lab coat.
Let’s not pretend this is about innovation. The R&D budget for ‘new’ drugs is a tiny fraction of what’s spent on patent litigation and marketing. The industry spends $30B annually on ads and $15B on legal teams. R&D? $12B.
So we’re celebrating a business model that prioritizes legal obstruction over scientific discovery. And we wonder why trust in institutions is collapsing.
It’s not a bug. It’s the feature.
If you’re on a brand-name drug that’s been around for over a decade and there’s no generic, ask your pharmacist for the active ingredient. Then Google it. Chances are, you’re paying 10x for the same molecule.
Don’t be afraid to push back. Your doctor might not know. Your insurer might be in on it. But you? You hold the power to ask, ‘Why?’
That question-simple, direct, relentless-is how change starts.